- Home
- News Centre Resources
- Shaping the future of R&D with real world insights into rare disease
Shaping the future of R&D with real world insights into rare disease

Written by: Christophe Hotermans, SVP, Head of Global Medical Affairs
Studying a rare disease often involves working with fragmented information, much like piecing together scattered pages of different books, each offering part of the story but rarely forming a complete picture. Researchers face this challenge due to small, often underdiagnosed, patient populations and limited understanding of how these diseases progress over time.

Now imagine gathering those scattered pages into a shared book—that grows with each patient visit, each data point, each new insight—into a more complete story. That’s the power of a patient registry.
A patient registry is a voluntary, observational study that collects health information during routine care, and is often established as a post-marketing regulatory requirement for approved treatments to monitor long-term outcomes in real-world settings.¹ These registries can go beyond tracking treatment responses to help advance the clinical understanding of rare diseases—the variability in how a rare disease presents and progresses, understanding changes in biomarkers, and insights into accelerating diagnosis—helping to identify unmet therapeutic needs. What makes registries powerful is not just the individual entries, but how they come together over time to tell a collective patient story.
In rare disease research, where data is often scarce and dispersed, these registries become an essential tool. They help inform how diseases are defined and diagnosed and open new avenues for therapeutic innovation. People living with rare diseases have collectively contributed to a shared evidence base that is reshaping how we research, design and develop potential new treatments and support others with these conditions.
A Decade of Discovery in HPP
One such example is the Global HPP Registry (NCT02306720), sponsored by Alexion, AstraZeneca’s Rare Disease Unit. HPP is a rare, inherited and progressive metabolic disease characterized by defective mineralization (the process that hardens and strengthens bones and teeth), impaired calcium and phosphate regulation, and non-skeletal manifestations, such as muscle weakness, generalized fatigue and pain.²⁻⁴ Because the condition presents differently across individuals and age groups, gathering consistent data at a large scale is challenging.
Established over a decade ago, the HPP Registry was the first international effort of its kind dedicated to studying HPP, creating a foundational resource for understanding this rare disease across diverse populations. Through the registry, data collected from patient volunteers from across medical centers and countries is pooled into one accessible source, making it possible to study a more diverse and representative patient population.
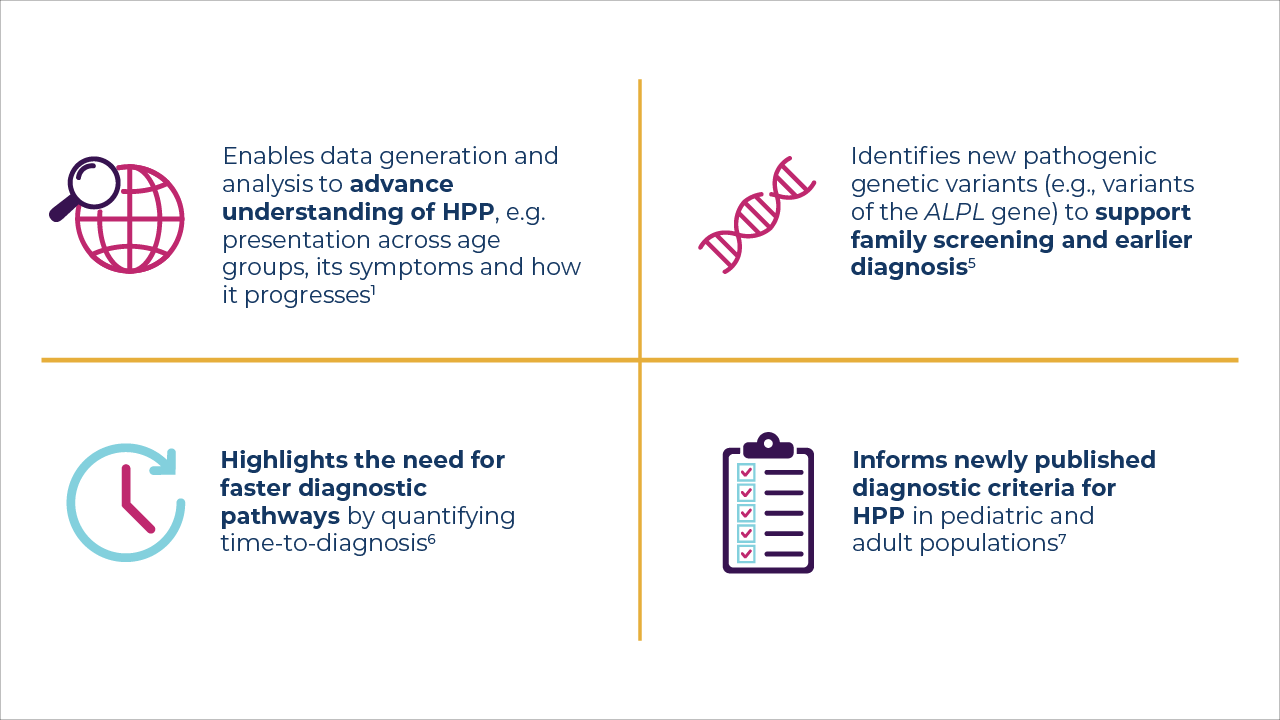
Over the past 10 years, insights from the Global HPP Registry have helped advance research and lay the groundwork for better and more personalized care, which:
A Foundation for the Future
Today, the registry serves as a vital foundation for ongoing advancements in the field for HPP, continuing to inform scientific research and clinical development, and how patients are diagnosed, treated and supported. The collective real-world evidence generated from this registry not only enriches current clinical trial data but is helping to advance studies on the next generation of therapies.
As we mark the 10th anniversary of the HPP Registry, we celebrate the impact it has generated and the community behind it, including patients, families, clinicians and researchers. Because when we can see the whole story—page by page, person by person—we can shape a better future for those living with this rare disease.
Understanding HPP
References
- HPPRegistry.com. Hypophosphatasia (HPP) Registry Website. Available here. Accessed September 2025.
- Rockman-Greenberg C. Hypophosphatasia. Pediatric Endocrinology Reviews. 2013;10(2):380-388.
- Dahir KM, et al. Clinical profiles of treated and untreated adults with hypophosphatasia in the Global HPP Registry. Orphanet J Rare Dis. 2022;17(1):277.
- Seefried L, et al. Burden of illness in adults with hypophosphatasia: data from the Global Hypophosphatasia Patient Registry. J Bone Miner Res. 2020;35(11):2171-2178.
- Kishani PS, et al. New insights into the landscape of ALPL gene variants in patients with hypophosphatasia from the Global HPP Registry. Am J Med Genet A. 2024;194(11):1-14.
- Khan AA, et al. Hypophosphatasia diagnosis: current state of the art and proposed diagnostic criteria for children and adults. Osteoporos Int. 2024;35(3):431-438.
- Brandi ML, et al. The challenge of hypophosphatasia diagnosis in adults: results from the HPP International Working Group Literature Surveillance. Osteoporos Int. 2020;35(3):439-449.
Share This Page
More articles you may like

Article • February 03, 2026
Advancing Policy, Partnerships and Progress for the Rare Disease Community
As we reflect ahead of Rare Disease Day, we reaffirm our commitment to pioneering innovations for the rare disease community and to shortening the time to diagnosis, improving access to lifesaving care, and advancing health care policies that support patients and families.

Diagnostics • September 17, 2025
Genomic Innovation to Support Newborn Screening of Rare Diseases
Today, while more than 10,000 rare diseases are known, recommended newborn screening panels cover only about 40 core conditions. This gap underscores the need for innovative approaches to expand the reach of newborn screening.

Thought Leadership • June 11, 2025
Paving Regulatory Pathways in Rare Disease
Many rare disease medicines are often a first of their kind, and a clear roadmap for their development and approval does not exist. Navigating the complex and sometimes unprecedented regulatory environment for rare disease requires innovation, collaboration and a patient-first mindset.

Media [News] • April 10, 2025
Advancing Rare Disease Research Through Patient-Centric Trial Design
As Head of Development, Regulatory and Safety at Alexion, Gianluca Pirozzi channels his life’s experience with rare disease to inform his work at Alexion, ensuring clinical programmes are fueled by a patient-focused mindset.

Article • February 24, 2025
Supporting Patients on Rare Disease Day, and Every Day
Rare Disease Day recognizes the 400 million people worldwide – and 30 million in the United States – who are living with a rare disease. While significant progress has been made in understanding rare diseases and developing treatments, the need for continued awareness, research and support is as great as ever.

Patient Support • February 05, 2025
Addressing the Unmet Needs of the Amyloidosis Community
We spoke with Cristina Quarta, Executive Medical Director, about a group of rare diseases known as amyloidosis, the medical needs within this community and how her work as a cardiologist shapes her approach to research and development (R&D) for rare cardiac conditions.

Patient Support • September 23, 2024
Shortening the Path to Diagnosis in Rare Disease
With 10,000 known rare diseases defined today, the path to diagnosis can often resemble a puzzle, with physicians as detectives searching for clues. Many physicians never encounter a rare disease beyond the pages of their medical school textbooks.

Research and Development • September 18, 2024
Getting to know Nick France, an Alexion R&D Leading Voice
To create meaningful advances in rare disease research, it takes an innovative culture, strong collaboration, and a patient-centric vision for delivering transformational solutions. At Alexion, these pillars are important to successfully develop impactful medicines for patients.

Patient Support • June 25, 2024
Getting to know Chathuri Daluwatte, an Alexion R&D Leading Voice
Artificial Intelligence (AI) is rapidly advancing and has the potential to have a transformative impact on clinical research and development (R&D).
Veeva ID: US/UNB-H/0753
Date of Preparation: October 2025
